Abstract
Background
Clonal abnormalities of the short arm of chromosome 12 (Abn12p) are seen in 3~5% of patients with acute myeloid leukemia (AML). Anecdotal reports associate these aberrations with adverse disease prognosis; however, no large studies have been published to formally document its clinical impact on prognosis, especially in patients undergoing allogeneic hematopoietic cell transplantation (HCT). This study aimed to test the hypothesis that AML patients with Abn12p have an inferior survival outcome after HCT. Here we present data from 110 patients with Abn12p, to contrast with 442 comparable AML patients but without Abn12p, all who have received HCT at Fred Hutch.
Patients and Methods
We performed a retrospective comparative cohort analysis based on available data from the Gateway database at Fred Hutch. Inclusion criteria for the testing cohort was AML patients diagnosed according to the current WHO criteria with any chromosome 12p abnormality by karyotype analysis who underwent a HSCT at Fred Hutch. A comparison cohort of AML patients without 12p abnormality was identified based on the following matching criteria: patient age difference within 5 years, transplant time difference within 2 years, conditioning intensity, and donor type. Four matching patients were identified for each Abn12p patient. Overall survival (OS), cumulative incidence of relapse (CIR) and non-relapse-mortality (NRM) after HCT were analysed for the whole cohort and for patients receiving HCT in first complete remission (CR1).
Results
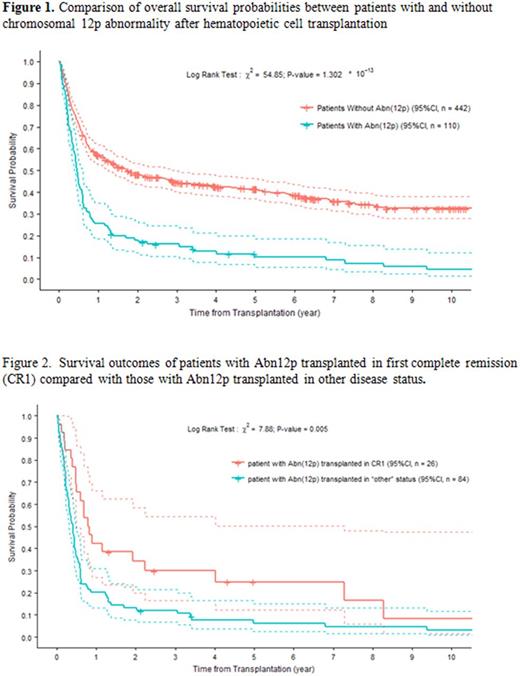
Data from 552 patients with full karyotype information were analysed. The median age was 50 years with a range from 2 years to 80 years. Twenty-seven patients were younger than 18 years. Patients transplanted in CR1 accounted for 32% (=177/552), slightly lower among Abn12p patients than among the non-Abn12p patients (24% vs. 34%, P=0.045). 450 patients (82%) were treated with standard myeloablative conditioning (MAC) regimens while the remaining patients received reduced intensity conditioning (RIC). Donors were matched related in 38% (including 4% haploidentical), matched unrelated donors in 36%, and partially matched or mismatched unrelated donors in 14% of the patients. Thirty-nine patients received unrelated cord blood. With a median follow-up of 12 months (range from 0 to 370 months), the 5-year OS probabilities were 12% for the Abn12p patients and 41% for the non-Abn12p patients (Figure 1. Hazard ratio 2.33, P<0.0001, and 2.28 after adjustment for pre-transplant disease status). Patients transplanted in CR1 showed better outcome even among those with Abn12p. The 5-year survival probabilities were 25% for the Abn12p patients among those transplanted in CR1 and 6% for the other Abn12p patients (Figure 2, P=0.005). The primary cause of treatment failure was relapse. Cumulative incidence of relapse at three years was 29% and 56% for patients with and without Abn12p, respectively (P<0.0001). NRM was not significantly different between the two groups. Different types of 12p abnormalities - translocation, deletion, additional material of undefined origin, monosomy, and inversion - showed similar clinical associations with mortality and relapse. Co-variation analysis of cytogenetic abnormalities showed that complex and monosomal karyotypes were present in 82% and 60% of Abn12p patients, respectively, compared with 19% and 7% of non-Abn12p patients, respectively (both P-values < 0.0001). Abnormal chromosome 17p abnormalities were seen in 37% of Abn12p patients compared with 5% of non-Abn12p patients (P<0.0001). ELN classification of the Abn12p patients showed 85% unfavorable cytogenetics, 14% intermediate, and 2% favorable compared with 24%, 72%, and 4%, respectively, among non-Abn12p patients (P<0.0001).
Conclusion
In conclusion, AML patients with Abn12p have a poor outcome after HCT. Abn12p tends to be present as part of a complex karyotype and hence considered unfavorable per ELN classification. Abn12p also overlaps with monosomal karyotype, but less so with 17p abnormalities. HCT in first complete remission still remains the treatment of choice for these patients. Clinical trial protocols should be considered for patients with Abn12p whenever possible.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal